Simulation study of oxytetracycline contamination remediation in groundwater circulation wells enhanced by nano-calcium peroxide and ozone

Material characterization
The morphological structure of the nCaO2
The SEM image of nCaO2 is shown in Fig. 2. The particle size and morphology of the nanoparticles can be clearly observed by electron microscope images.
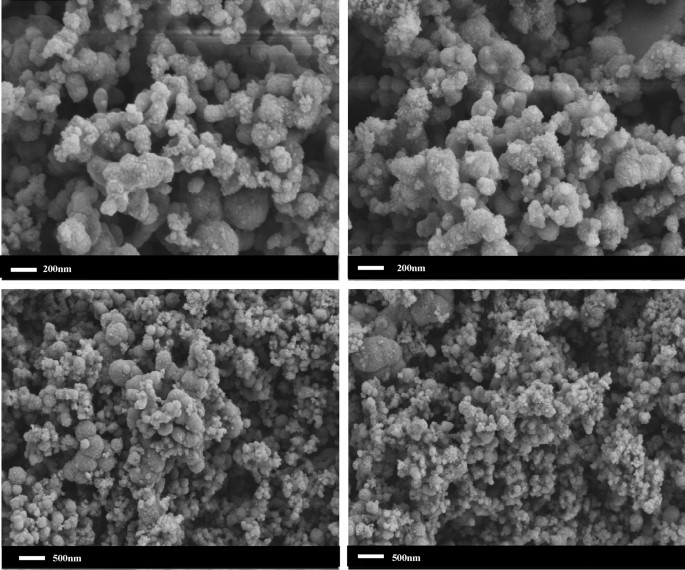
The SEM image of the nCaO2.
Calcium chloride and hydrogen peroxide are used as the main materials, and CTMAB is used as the dispersant. The characterization results show that when the average diameter of the prepared nanoparticles is between 80 and 150 nm, nCaO2 accords with the particle size of nano-sized materials, and the particles are uniformly dispersed and there is no agglomeration.
Analysis of the main components of nCaO2
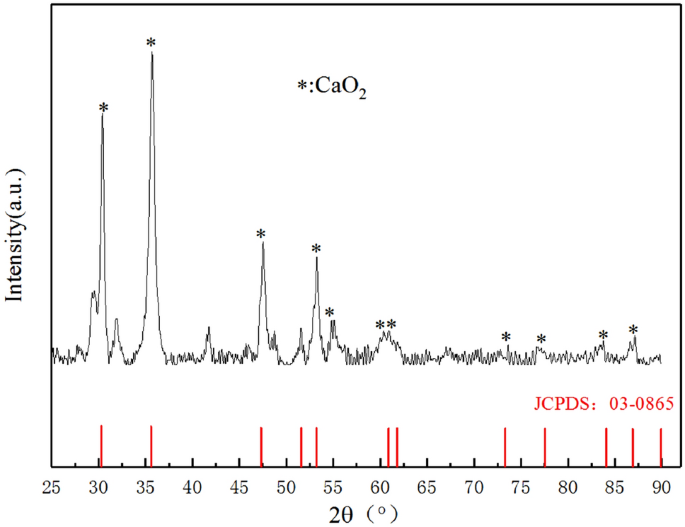
Whether the material is CaO2 can be qualitatively prepared by X-ray diffraction (XRD), and the effect of the added dispersant on the main structure and composition of the particles can be analyzed. It can be confirmed by Fig. 3 that there are characteristic peaks at 2θ of 30.4°, 35.73°, 47.48°, 51.56°, 53.24°, 60.89°and 61.78°. Compared with the standard card of CaO2, it is found that the positions of the diffraction peaks are the same, indicating that the main components are the same. The standard line is not a smooth straight line, indicating that there are a small amount of impurities in the prepared material, but it will not affect the properties of nCaO2.
Optimization of degradation conditions
By varying one variable at a time in beaker experiments, it was concluded that a starting concentration of 20 mg/L of Oxytetracycline, 0.25 g/L of calcium peroxide solution and 8 mg/L of O3 at laboratory room temperature, with the solution pH on the alkaline side, was the most effective in degrading Oxytetracycline.
Strengthen groundwater circulation wells to repair OTC contaminated groundwater
NCaO2-assisted GCW experimental study
The OTC concentration in the sand box at different aeration times is shown in Fig. 4. The horizontal axis is the length of the sand box, and the vertical axis is the height from the bottom of the sand box. The circulation well is installed at the center line of the sand box.
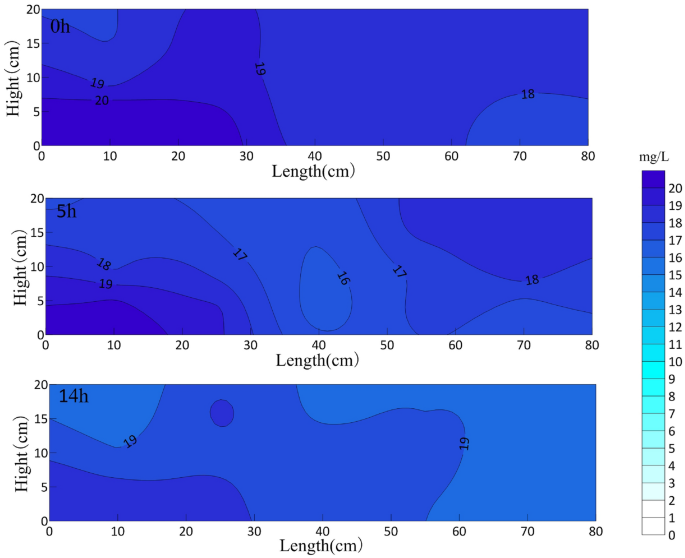
OTC concentration distribution in sand box with different aeration time (mg/L).
After 14 h of aeration, the average concentration of oxytetracycline in the sand box decreased from the initial 17.35 mg/L to 15.72 mg/L, and the degradation rate was only 8.9%. The possible reason is that nCaO2 is difficult to dissolve in water, and the granular media in the aquifer will affect the diffusion of nCaO2, even the pores of the media will absorb a small amount of nano-calcium peroxide particles, and the oxytetracycline itself has a stable structure. However, under the influence of aeration, nano-calcium peroxide in aquifer flows into the whole box with the circulation of water, and oxytetracycline is distributed more evenly in the sand box under the impetus of circulation.
O3-assisted GCW experimental study
With the increase of aeration time, the OTC concentration in the whole sand box decreases significantly. From a horizontal point of view, the closer to the circulation well, the higher the OTC degradation efficiency, and vice versa. From the vertical point of view, OTC in the upper part of GCW is degraded first, then in the middle part, and the lowest efficiency is in the lower part of the circulation well. It can be clearly seen that the repairing area of the circulation well is an approximate conical area with the centerline of GCW as the axis, and the oxytetracycline concentration in the whole box is symmetrically distributed.
Figure 5 shows the distribution of oxytetracycline in the sand box at different aeration times. It can be seen from the figure that the average concentration of oxytetracycline decreased to 12.62 mg/L in the first 2 h of the reaction, which decreased by 36.8% compared with that before aeration, and the removal rate was 0.061 mg/min. With the aeration experiment, the removal rate of oxytetracycline decreased slowly within 2–10 h of cumulative aeration, and after 10 h of cumulative aeration, the average concentration of oxytetracycline will reach 5.16 mg/L, which is 74.17% lower than that before aeration. After aeration, the tailing concentration of oxytetracycline is 5.16 mg/L.
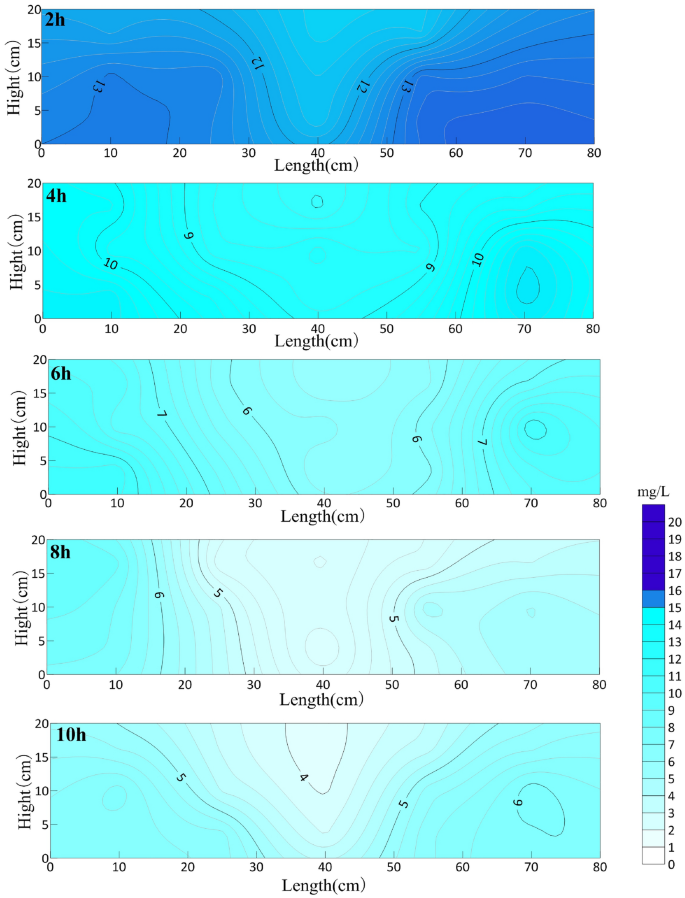
OTC concentration distribution in sand box with different aeration time (mg/L).
Experimental study on GCW enhanced by nCaO2and O3
As can be seen from Fig. 6, with the continuous aeration experiment, the concentration of oxytetracycline near the circulation well decreases the fastest, and it presents a symmetrical distribution centered on the aeration pipe in the circulation well. The concentration of OTC in the sand box decreases gradually, but the degradation efficiency is the highest in the area close to the circulation well, and the removal efficiency is the slowest in the area far away from the circulation well, that is, on both sides of the sand box. In the first 2 h of the reaction, the average concentration of oxytetracycline decreased to 10.93 mg/L, which was 45.35% lower than that before aeration, and the removal rate was 0.076 mg/min. With the aeration experiment, the removal rate of oxytetracycline decreased slowly. After 10 h of aeration, the average concentration of oxytetracycline reached 3.4 mg/L, which was 83% lower than that before aeration. After aeration, the remediation entered the tailing stage, and the tailing concentration of oxytetracycline was 3.4 mg/L. Oxytetracycline is a refractory organic substance, and the presence of media with different particle sizes in the aquifer will hinder the contact between O3 and pollutants, thus affecting the oxidation efficiency. Through the analysis of the experimental results, when circulation well technology is used to remediate refractory organic substances, it will achieve better remediation effect when combined with other oxidation technologies.The groundwater in Daqing area is weakly alkaline, and the experimental effect was the most ideal. The nCaO2 content added in this experiment was less, and the pH value of the solution before and after the experiment was small, which had little effect on the solution.
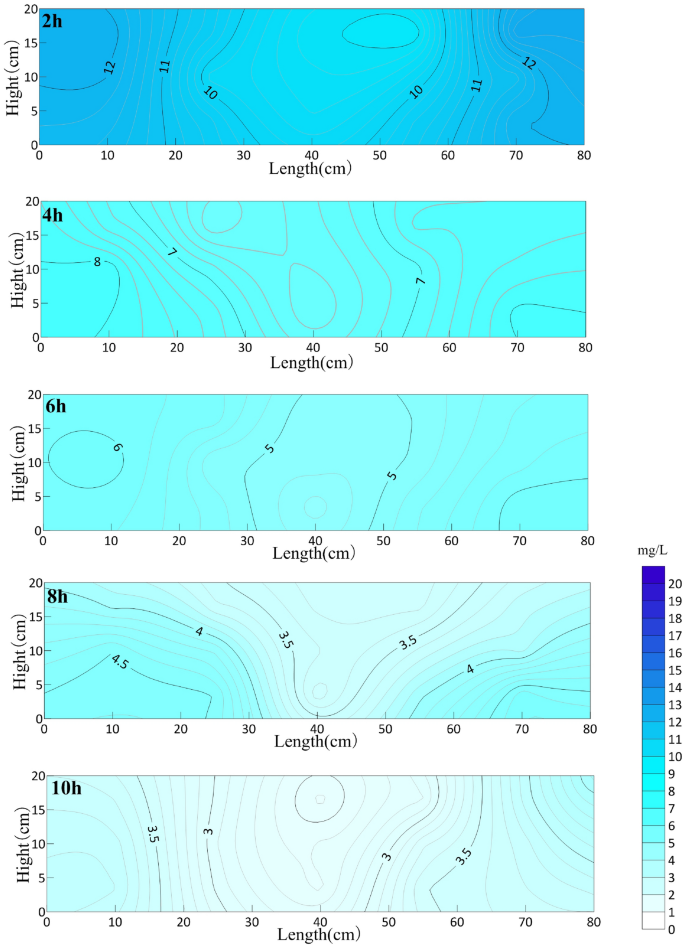
OTC concentration distribution in sand box with different aeration time (mg/L).
After 10 h of cumulative operation of GCW, the average removal rates of OTC in sand box aquifer are 74.2% and 83.3% under the action of O3 alone and nCaO2 in cooperation with O3. The highest removal rates in aquifer are 80.46%(C3) and 88.13%(C3), and the lowest removal rates are 69.31%(B5) and 76.81%(A5). The highest removal rate was only 0.5% and 0.3% higher than that of aeration for 10 h. After that, the tail concentration was 5.16 mg/L and 3.4 mg/L in the stable stage of the experiment, which indicated that nCaO2 combined with O3 enhanced the OTC degradation compared with O3 alone.
The variation of OTC concentration after stopping aeration
At 0 h, 3 h, 6 h, 9 h, 12 h and 15 h after the experiment of O3 assisted circulation well repair technology, the average concentration of oxytetracycline in the simulated sandbox was 5.16 mg/L, 5.46 mg/L, 6.13 mg/L, 6.75 mg, 7.33 mg/L and 7.45 mg/L. Because in the aeration experiment, ozonation oxidizes part of the oxytetracycline dissolved in the aqueous phase, because the surface of the sand is rough and the specific surface area is very large, part of the oxytetracycline in the solution is adsorbed. The oxytetracycline adsorbed in the interstitial space of the sand desorbed and gradually transferred to the liquid, resulting in an obvious rebound in the concentration of oxytetracycline in the solution.
The average concentration of oxytetracycline in the sandbox was 3.4 mg/L, 3.25 mg/L, 3.12 mg/L, 3.05 mg/L, 3 mg/L and 2.96 mg/L after the repair experiment of nCaO2 combined with O3 enhanced circulating well. It can be seen that the concentration of oxytetracycline in the sandbox decreased slowly after the end of the experiment. The reason is that after the end of the experiment, the nCaO2 particles that remained in the sandbox did not participate in the reaction slowly released H2O2 in the water. Catalytic O3 molecules produce more oxidizing hydroxyl radicals, oxidize oxytetracycline in water, and spread to the whole simulation box with the flow of groundwater. The experimental results show that nCaO2 combined with O3 in-situ remediation technology can eliminate the rebound of pollutants in in-situ remediation, expand the repair area and prolong the repair time. And the pH value of the solution changed little before and after the experiment, which had little effect on the pH value of the solution.













